🧪 Cubic Equations of State Calculator
Calculate thermodynamic properties using Peng-Robinson and SRK equations
🔬 Component Properties
📊 Calculated Properties
Enter parameters and click “Calculate Properties” to see results.
⚗️ Mixture Properties
📈 Mixture Results
Enter parameters and click “Calculate Mixture” to see results.
📚 Cubic Equations of State Theory
General Form:
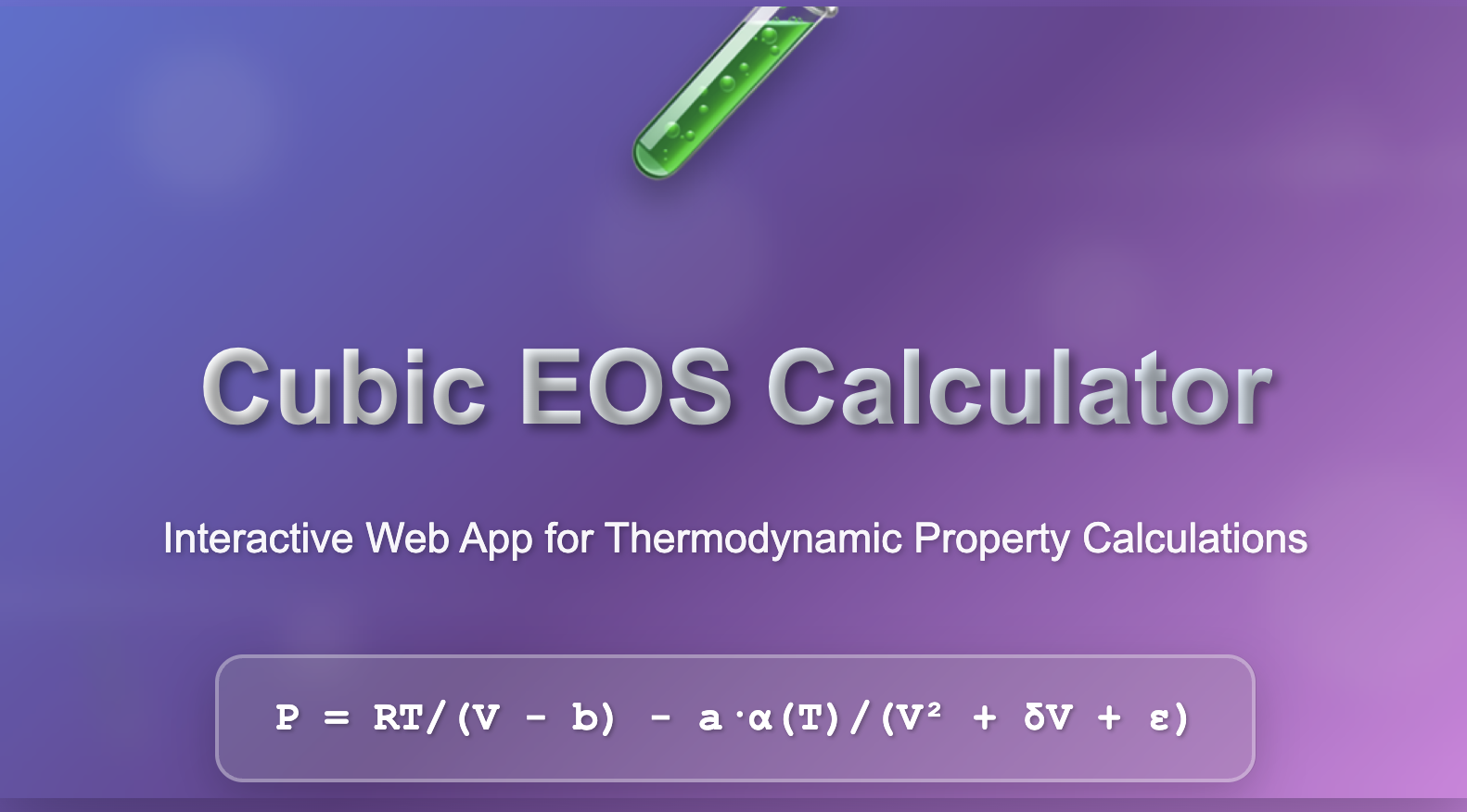
P = RT/(V – b) – a·α(T)/(V² + δV + ε)
Peng-Robinson (PR) Parameters:
δ = 2b, ε = -b²
a = 0.45724·R²Tc²/Pc
b = 0.07780·RTc/Pc
α = [1 + κ(1 – √(T/Tc))]²
κ = 0.37464 + 1.54226ω – 0.26992ω²
a = 0.45724·R²Tc²/Pc
b = 0.07780·RTc/Pc
α = [1 + κ(1 – √(T/Tc))]²
κ = 0.37464 + 1.54226ω – 0.26992ω²
Soave-Redlich-Kwong (SRK) Parameters:
δ = b, ε = 0
a = 0.42748·R²Tc²/Pc
b = 0.08664·RTc/Pc
α = [1 + κ(1 – √(T/Tc))]²
κ = 0.480 + 1.574ω – 0.176ω²
a = 0.42748·R²Tc²/Pc
b = 0.08664·RTc/Pc
α = [1 + κ(1 – √(T/Tc))]²
κ = 0.480 + 1.574ω – 0.176ω²
Key Applications:
- Process Design: Vapor-liquid equilibrium calculations
- Phase Behavior: Critical point determination
- Property Estimation: Fugacity and departure properties
- Mixture Calculations: Using Van der Waals mixing rules
Note: This calculator provides educational demonstrations.
For production use, employ specialized thermodynamic software or libraries.
📊 Visualization
Interactive P-V diagram will be displayed here after calculations